THE ATOM Lesson 1 Check for Understanding
Atomic Structure Practice
Part ONE: Terminology - Explain how to find the following atomic properties
Atomic Number: position on the periodic table - bottom number same as protons
Protons: same as atomic number
Neutrons: Mass number - atomic number
Electrons: if atom is neutral then same as protons
Charge: protons - electrons
Mass Number: protons + neutrons
Part TWO: Notation - Using the terms from above, label/ annotate each notation type below
Hyphen Notation:
Examplenium - A
Nuclear Symbol:
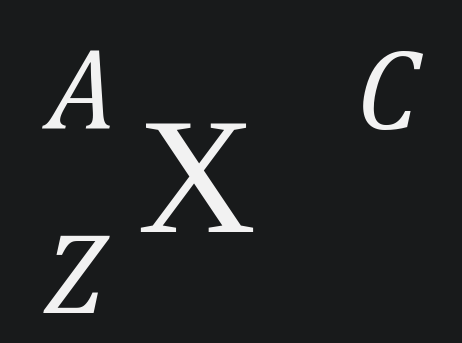
Part THREE: Notation - Fill in the blank boxes with the correct information. Circle the numbers that are isotopes. Highlight the ions.
| # | Element Hyphen Notation | Nuclear Symbol | Atomic Number (Z) | Mass Number (A) | # Protons | # Neutrons | # Electrons |
|---|---|---|---|---|---|---|---|
| 1 | Hydrogen – 1 | 1 | 1 | 1 | |||
| 2 | 7 15N | 7 | 8 | ||||
| 3 | Aluminum – 27 | 27 | 13 | ||||
| 4 | 36 | 45 | 36 | ||||
| 5 | Uranium – 238 | 92 | 146 | ||||
| 6 | Helium – 4 | 2 | 4 | ||||
| 7 | 2 5He | 2 | 5 | ||||
| 8 | Silicon – 28 | ||||||
| 9 | 38 | 51 | |||||
| 10 | 77 | 196 | |||||
| 11 | Lithium – 7 (+ 1 cation) | ||||||
| 12 | 74 184W | 184 | |||||
| 13 | 54 | 79 | 54 | ||||
| 14 | Magnesium – 25 (+2 cation) | 25 | |||||
| 15 | 12 | 6 | |||||
| 16 | (-2 anion) | 82 | 34 | ||||
| 17 | Antimony – 123 (-3 anion) | ||||||
| 18 | 25 55Mn | ||||||
| 19 | Mercury – 208 | ||||||
| 20 | (+3 cation) | 197 | 79 |