THE ATOM Lesson 2 Check for Understanding
Part ONE: Working with Isotopes
Directions: Use the information provided and your Reference Tables to complete the table. Assume all atoms are neutral.
| Isotope Notation | Name | Mass Number | Atomic Number | # Protons | # Neutrons | # Electrons | |
|---|---|---|---|---|---|---|---|
| 1. | 136C | Carbon-13 | 13 | 6 | 6 | 7 | 6 |
| 2. | 216K | Potassium-40 | 21 | 19 | 19 | 22 | 19 |
| 3. | 2210 Ne | Neon-22 | 22 | 10 | 10 | 12 | 10 |
| 4. | 178 O | Oxygen-17 | 17 | 8 | 8 | 9 | 8 |
| 5. | 6430Zn | Zinc | 64 | 30 | 30 | 34 | 30 |
6. Define Isotopes. isotopes are atoms of the same element that have the same number of protons but a different number of neutrons or mass number
- Which atoms (if any) in the table above are isotopes of each other?
None, they all have different atomic numbers / amounts of protons. They are not atoms of the same element.
8. Which of the following has the smallest mass?
- Proton b. Electron c. Neutron d. Their Masses Are Equal
Electron
9. The letter “Y” in the diagram marks the (check ALL that apply):
-
Location of the Protons
-
Nucleus
-
Mostly Empty Space
-
Location of the Neutrons
-
Location of the Electrons
-
Region of Greatest Density
-
Of the basic atomic particles, the one that would be attracted to a negatively charged metallic plate is the:
-
Electron
-
Neutron
-
Proton
Part TWO:Calculating Average Atomic Mass round all answers to 2 decimal places
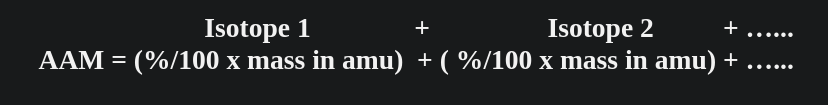
-
Calculate the average atomic mass of bromine. One isotope of bromine has an atomic mass of 78.92amu & a relative abundance of 50.69%. The other major isotope of bromine has an atomic mass of 80.92amu & a relative abundance of 49.31%.
show your work
(78.92 * .5069) + (80.92 * .4931 ) 79.9062 79.91
-
Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.78% has a mass of 32.971amu and 4.22% have a mass of 33.967amu.
show your work
(31.972 * .9500) + (32.971 * .0078 ) + (33.967 * .0422) 32.0639812 32.06
- The average atomic mass for gallium is 69.723 amu. What is the mass of the lighter isotope if Ga-71 (70.9249 amu) has a percent abundance of 39.6 %?
show your work
69.723 = (70.9249 * .396 ) + ( x * .604 )
69.723 = (28.0862604) + (x * .604)
41.6367396 = x * .604
68.9349993377 = x
^ ( i did 41.6367396 / .604 here. just solving like a normal equation.)
68.93
- Conceptual: No calculation needed.
There are three isotopes of silicon. They have mass numbers of 28, 29 and 30. The average atomic mass of silicon is 28.086amu. Which isotope has the highest % abundance and which has the lowest?
Highest 28 Lowest 30
Answers Part TWO: 11) 79.91 amu 12) 32.06 amu 13) 68.93 amu