L1 Energy & Specific heat
Energy is efined as the ability to do work or transfer heat energy
potential energy is energy at rest due to the position of an object, chemical potential energy is stored in the substance’s bonds
kinetic energy is the energy of the motion of particles in a substance and is directly proportional to kelvin temperature.
kinetics is defined as the study of how fast a reaction occurs
we can measure how fast or slow a reaction takes place by looking at its reaction rate: Rate = change in amount of substance / change in time
Collision theory: these 3 conditions MUST be met for a reaction to occur
- Reactants must collide
- Collisions must be at the correct orientation
- Collisions must meet a minimum energy called Activation energy for the reaction to occur
Factors which affect reaction rate:
- Nature of Reactant: Some substances are just more reactive than others
- Surface area: as surface area increases, more effective collisions happen & rate increases
- Concentrations of the reactants: as reaction concentration increaess, more effective collisions happen & the reaction rate increases. ( More reactants mean greater collision frequency )
- Temperature: Molecules at a higher temperature have higher average speed so they move faster with greater collision frequency. Also, reactants must have minimal energy ( activation energy ) to collide. At higher temperatures, collisions have more energy, which contributes to required activation energy
- Catalyst: substance that speeds up the rate of reaction without being used up.
- Enzymes are catalysts in the body
- Catalysts lower activation energy by letting it proceed in a different way
- Lower Ea ( activation energy ) = faster reaction
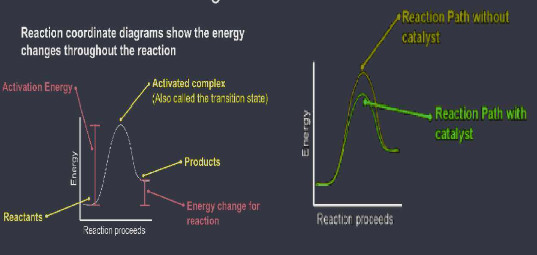
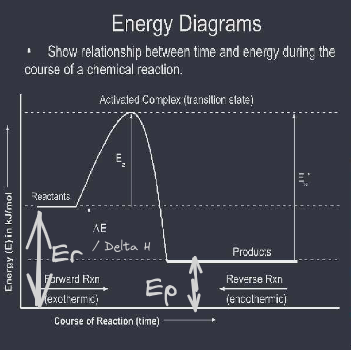
Heat of reaction ( deltaE ) ( Enthalpy change ) can be:
- Endothermic ( delta E (delta H)) is positive: products have higher energy than reactants
- Exothermic: (deltaE (deltaH)) is negative; products have lower energy than reactants To calculate deltaH ( heat of the raction or change in enthalpy): subtract the final - initial energies; dependent on which direction you are looking at
Forward direction deltaH deltaE = Eproducts + Ereactants
Thermochemical Equation: Balanced chemical equations that show the enthalpy change released or absorbed in a chemical reaction Enthalpy: heat released or absorbed during a chemical reaction; classified as positive or negative
HOT PACK: an exothermic reaction is when the system releases energy; heat flows INTO a reaction and the surroundings get CLOSER deltaH will be negative
COLD PACK: An endothermic reaction is when the system absorbs energy, heat flows into a reaction and the sruroundings get colder deltaH will be positive
Stoichiometry and enthalpy: Once the deltaH is determined or given, it can be used in stoichiometric calculations Use moles ( coefficients ) of reactants or products and the deltaH as a conversion factor
if you are solving for deltaH, you must use the sign. If you are solving for how much energy is absorbed or released, you do not have to put the sign.
a calorie is defined as the amount of heat needed to raise the temperature of 1g by 1C ( 1 cal = 4.184 J ) most common units are Joules or kilojoules
specific heat is the amount of heat required to raise the temperature of 1g of a substance by 1C
specific heat ( c ) = heat in joules / ( mass ( m ) * change in temp ( deltaT) )
different substances have different specific heats water has a specific heat of 4.184 J/gC. Iron has a specific heat of .4490 J/gC. Gold has a specific heat of .129 J/gC The higher the specific heat the more energy it takes to change its temperature
q = heat ( joules ) m = mass ( g ) c = specific heat ( J/gC) deltaT = change in temp
L2 Heat Calculations & Properties of Solids and Liquids
to calculate heat:
q = m * C * deltaT
Properties of a Liquid:
particles more spread out than solid, somewhat close packing
particles are free to move past eachother; flow
incompressible
weaker attractions
variable shape and definite volume
properties of a solid:
closest packing of molecules
vibrate in place; low kinetic energy of particles
incompressible
strong attractive forces
definite shape and definite volume
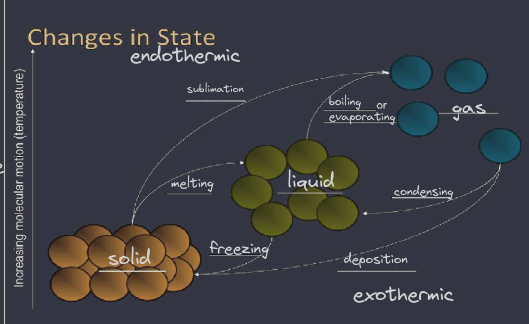
Endothermic:
Kinetic energy must be put INTO the substance in order to increase the motions of the molecules so as to break the intermolecular forces holding the particles together
melting: change of state from a solid to a liquid
vaporization ( boiling or evaporation) : change of state from a liquid to a gas
sublimation: direct change of state from a solid to a gas
Exothermic:
Kinetic Energy must be taken OUT ( removed ) from the substance for the molecules to slow down so that the intermolecular forces can begin to hold the particles together
Freezing: change of state from a liquid to a solid
Condensation: change of state from a gas to a liquid
Deposition: direct change of state from a gas to a solid
Temperature of state Changes:
freezing point = melting point
condensation point is the temperature at which a gas turns into a liquid
boiling point = condensation point
all substances have their own specific freezing and boiling point, which makes this physical property a great way to identify an unknown substance
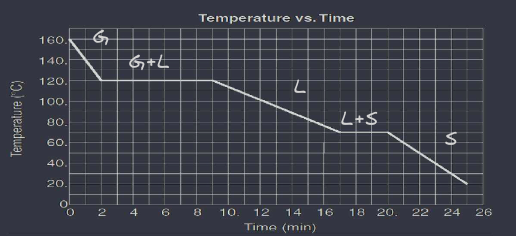
L3 Heating & Cooling Curves & Phase Diagrams
A heating / cooling curve is a diagram that shows how solids liquids and gasses change state when temperature is changed. Plateaus represent the changes of state. Slopes represent the pure states ( solid, liquid, or gas) at the plateaus, kinetic energy remains constant because temperature remains constant while potential energy changes
Phase diagrams show how solids liquids & gases change state as both temperature and pressure changes. Crossing a line between states determines the change of state ( boiling, melting, etc ) a point directly on a line will identify the pressure and temperature ( boiling point, melting point, etc ) of the phase change triple point is the temperature and pressure in which all 3 states coexist critical point is the temperature and pressure in which a gas can no longer liquefy
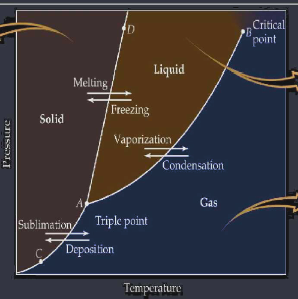
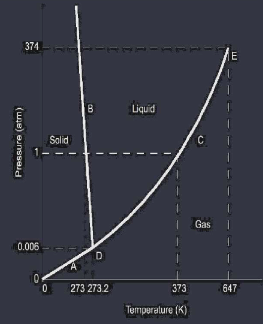
L4 Heat Calculations of Phase Changes
Measuring heat during phase changes:
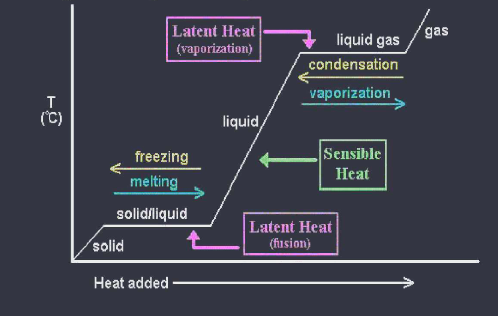
Latent heat of fusion ( deltaHfus ) is the heat energy required to melt one gram of a solid at its melting point. q = deltaHfus * mass
Latent heat of solidification ( deltaHsolid ) is the heat energy lost when one gram of a liquid freezes to a solid at its freezing point. q = deltaHsolid * mass
Latent heat of vaporization is the heat to vaporize one gram of a liquid at its normal boiling point. q = deltaH vap * mass
Latent heat of condensation is the heat energy released when one gram of a liquid forms from its vapor. q = deltaHcond * mass