L1 Definitions, Characteristics, and Idenification
An acid is a substance that produced hydrogen ions ( H+ ) when dissolved in water
the hydrogen ions immediately react with water to form the hydronium ion (H3O+)
to recognize an acid look for an aqueous covalent compound that has a hydrogen as the first element
5 big acids:
- HCL ( hydrochloric )
- HNO3 ( nitric )
- H2SO4 ( sulfiric )
- H2CO3 ( carbonic )
- HC2H3O2 ( acetic )
You can identify an Arrhenius base by looking for an ionic compound that contains a metal connected to a hydroxide ion, OR recognizing NH3 which is called ammonia
not all compounds ending in -OH are bases, the OH must be connected to a metal CH3OH is an alcohol
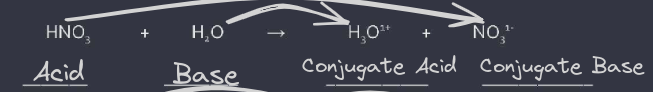
A Bronsted-Lowry acid is a substance that has a proton or hydrogen donor A Bronsted-Lowry base is a substance that has a proton or hydrogen acceptor
Conjugate acid and base pairs: found only on the right side of an equation Conjugate Bases are substances formed when a base loses an H+ ion Conjugate Acids are substances formed when a base gains an H+ ion
example:
Characteristics:
Acids:
- Examples: HCL HNO3 HBr
- Produce hydronium ions when dissolved in water
- Tastes sour
- Reactss with Metals to produce hydrogen ions
- Neutralizes a base to form a salt and water
Bases:
- Examples: NaOH Fe(OH)3 Ca(OH)2
- Produce OH-(hydroxide) ions when dissolved in water
- Tastes bitter
- Feels slippery
- Neutralizes an acid to form a salt and water
both form ions when dissolved in water. they are called electrolytes
L2: Strength vs Concentration & PH scale + Indicators
Strength is determined by how many ions are present Strong acids show all acid molecules seperating ( dissociating ) into hydrogen ions ( H+ ) and anions in water. ONLY ions are present.
Ex: HCL, H2SO4, HNO3
Weak acids show mostly acid molecules intact with only a few hydrogen ions (H1+) and anions present in water ( less than 5% of molecules dissociate into ions, mostly molecules are present). They are considered weak electrolytes.
Ex: H2CO3 or HC2H3O3
Concentration: determined by how much solute is dissolved in solvent
Look at whether there is a lot or little of the dissolved substance; do not look at ions vs molecules; the solute is the acid or base molecule; the solvent is the water.
Concentrated acid: Lots of acid ( solute ) is dissolved into water ( solvent ) Dilute acid: A little amount of acid ( solute ) is dissolved into water ( solvent )
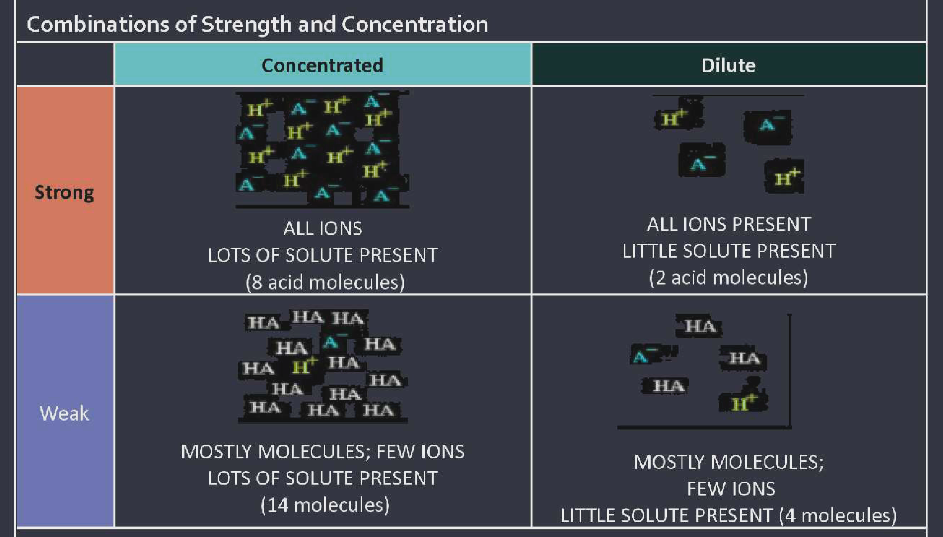
base strength and concentration follow the same patterns as acids.
The stronger an acid / base is, the more concentrated it is, and the more dangerous it is to you.
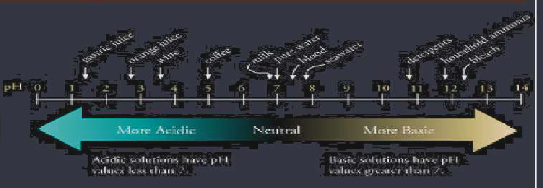
pH scale measures the acidity of a sample
Acids have a pH of less than 7 Bases have a pH of more than 7 Neutral solutions have a pH of exactly 7
indicators are substances that change color based on pH
liquid indicators include:
phenolphthalein, which stays colorless if it is added to an acid, and turns pink if added to a base
bromothymol blue, which turns yellow in an acid and blue in a base
paper indicators include: litmus and pH paper. pH paper is more precise, allows you to match to a specific ph number.
Litmus paper turns red if its in an acid, and blue if it is in a base.
L3: pH Concentrations
the formula for calculating pH is : pH = -log10[H3O+] square brackets mean molarity
the formula for calculating the concentration of H+ is [H3O+] = 10^-pH the lowest pH represents the highest concentration of the hydronium ion each time pH changes by 1, concentration of hydronium changes 10x
Water splits into ions on its own sometimes, which is called autoionization.
to calculate pOH use pOH = -log[OH-] to calculate OH concentration: [OH-] = 10^-pOH to relate pH and pOH: pH + pOH = 14
L4 Neutralization Reactions and Titrations
Neutralization reaction: when an acid and base react to form a slat ( ionic compound ) and water
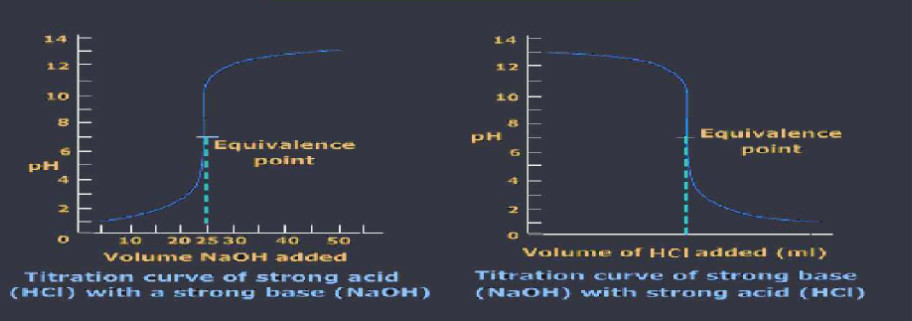
Titrations: a technique where the addition of a known volume a known concentration solution ( acid or base ) is reacted with a known volume of unknown concentration solution to determine the concentration of the solutions
The titrant is the known concentration in the buret, and the analyte is the known concentration in the flask. add an indicator to the analyte in the flask add titrant to the analyte drop by drop until you get a permanent color change end point is the point at which the indicator changes color, it signals the equivalence point and the stop of the titration equivalence point is when there are no reactants left over, they have all been reacted and completely used up. solution contains only products at this point. moles of acid = moles of base.
indicators are liquids that change color based on pH loevel, they are used to show when the endpoint has been reached. e.g. phenolphthalein and bromothymol blue. you are supposed to select a liquid indicator that has a pH range close to that of the pH of the equivalence point of the titration.
short cut formula : naMaVa = nbMbVb
Titration curve graphs show the changes of pH during a titration to identify pH of the equivalence point, take the vertical region and cut the length in half and then look to what pH value aligns to that point.