Unit 2 Project
As a team of 4 in your breakout group, complete the following parts for each of your assigned elements. Submit one document for your group.
You will break apart into 2 smaller groups and each subgroup will work together on each part step by step for one of the two assigned elements (The other small group will do the same with the second element).
-
It is suggested that each group member checks the other group’s work using the attached rubric before submitting the final document.
Group 1: Lithium and Gallium Group 5: __Chlorine__and Magnesium
Group 2: Vanadium and Silicon Group 6: Silicon and Boron
Group 3: Boron and Nitrogen Group 7: Carbon and Bromine Group 4: Potassium and Vanadium Group 8: Oxygen and Gallium
Part 1: Bohr Model (2 points)
- Draw a Bohr Model for your element placing all the electrons in the appropriate energy levels.
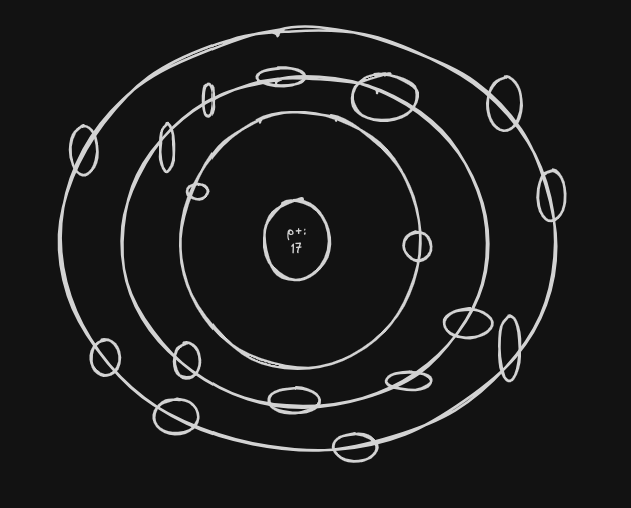
NOTE: Use Part 2 of U2 CFU1 as a guide. Draw the nucleus as a solid sphere. There is no need to add protons & neutrons. (1 point each)
-
How many valence electrons does your element have? (½ point each) 7
-
Draw a Lewis Dot Diagram for your element. (½ pt each)

Part 2: Electron Arrangement (4 points)
-
Write the ground state electron configuration for your element applying the Aufbau Principle learned in class. (1 point each)
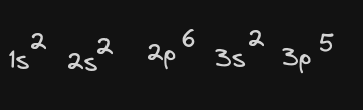
-
Draw the orbital notation for your atom. Be sure to:
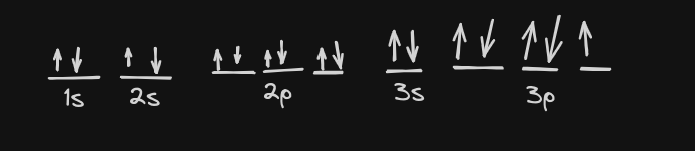
-
label your orbitals (.5 pts each)
-
apply both Hund’s Rule and Pauli Exclusion Principle (1 point each)
-
How many unpaired electrons are there? (.5 pts each) 1
-
Write the shorthand configuration (noble gas configuration) for your element.(1 point each) [Ne] 3s2 3p5
Part 3: Periodic Table Properties (7 points)
-
Determine the location of your element on the periodic table by stating its period number and its group number. (1 point each) Period #:____ Group #:____
-
Classify your element as either a metal, nonmetal or metalloid. Using your notes, state 2 general properties for the class you selected. (3 points: 1 point for the correct classification and 1 point each for a general property of that class of elements)
-
What is the family name of your element? Select from the following below: (1 point each)
| Alkali Metals | Alkaline Earth Metals | Transition Metals | Halogens |
|---|---|---|---|
| Noble Gases | Nitrogen Family | Boron Family | Oxygen Family (Chalcogens) |
| Carbon Family |
- Using your notes, state 2 properties specific to your family of elements. It cannot be the same properties you used above in 2b and must be specific to your family. (1 point each)
Note: If your element is in the Boron Family, Carbon Family, Nitrogen Family or Chalcogens, you will need to google properties of that family- they are not in your notes.
Part 4: Trends of the Periodic Table (27 points)
Period Trends (15 points) Note: Periods run horizontal (left to right) on the periodic table
-
Atomic Radius (AR) (6 points)
-
Define Atomic radius using the definition from your notes .(1 point)
-
Does the atomic radius increase or decrease when moving from left to right across the period?(1 point)
-
Explain your answer to 1b. You must include at least three of the following words used correctly in your explanation: proton OR nuclear charge, stronger OR weaker attractions and larger OR smaller size. (3 points)
-
Identify an element within the same period for your assigned element. State whether your assigned element’s atomic radius is larger or smaller than it. Copy this statement and fill in the missing blanks. My element has a _________ AR than __________. (1 point each)
-
Ionization Energy (IE) (6 points)
-
Define Ionization Energy using the definition from your notes. (1 point)
-
Does the ionization energy increase or decrease when moving from left to right across a period? (1 point)
-
Explain your answer to 2b. You must include at least three of the following words or phrases, used correctly, in your explanation: proton OR nuclear charge, stronger OR weaker attractions and more OR less energy. (3 points)
-
Identify an element within the same period for your assigned element. State whether your assigned element’s ionization energy is larger or smaller than it. Copy this statement and fill in the missing blanks. My element has a _______ IE than __________. (1 point each)
-
Electronegativity (EN) (3 points)
-
Define Electronegativity using the definition from your notes (1 point)
-
Does electronegativity increase or decrease when moving from left to right across a period? (1 point)
-
Identify an element within the same period for your assigned element. State whether your assigned element’s electronegativity is larger or smaller than it. Copy this statement and fill in the missing blanks. My element has a _______ EN than __________. (1 point each)
Group Trends (12 points)Note: Groups run vertical (up & down) on the periodic table.
-
Atomic Radius (AR) (5 points)
-
Does the atomic radius increase or decrease when moving from top to bottom down a column?(1 point)
-
Explain your answer to 1a. You must include at least three of the following words & phrases, used correctly, in your explanation: energy level OR closer OR farther distance from nucleus, stronger OR weaker attractions and larger OR smaller size. (3 points)
-
Identify an element within the same group for your assigned element. State whether your assigned element’s atomic radius is larger or smaller than it. Copy this statement and fill in the missing blanks.My element has a ______ AR than _____________. (1 point each)
-
Ionization Energy (IE) (5 points)
-
Does the ionization energy increase or decrease when moving from top to bottom down a column?(1 point)
-
Explain your answer to 2a. You must include at least three of the following words & phrases, used correctly, in your explanation: energy level OR closer OR farther distance from nucleus, stronger OR weaker attractions and more OR less energy. (3 points)
-
Identify an element within the same group for your assigned element. State whether your assigned element’s ionization energy is larger or smaller than it. Copy this statement and fill in the missing blanks. My element has a _________ IE than _________. (1 point each)
3. Electronegativity ( 2 points)
-
Does the electronegativity increase or decrease when moving from top to bottom down a column? (1 point)
-
Identify an element within the same group for your assigned element. State whether your assigned element’s electronegativity is larger or smaller than it. Copy this statement and fill in the missing blanks. My element has a _______ EN than __________. (1 point each)