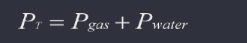L1: Properties of Gases and Kinetic Molecular Theory
Properties of gases:
- Made of atoms or molecules that are far apart from eachother ( weak intermolecular forces )
- High kinetic energy
- Lowest density of the 3 states
- Gases take the shape and volume of their container
- Can expand
- Can Compress
Kinetic Molecular Theory is based on theoreitcal ideal gases that follow the assumptions it makes
Here are those assumptions:
- Gases are made of that are in chaotic motion
- Kelvin temperature of a gas is directly proportional to average kinetic energy ( as temp goes from 100K to 200K average kinetic energy doubles. factor of 1.08 for celcius. ( 25 - > 50))
- Gas particles do not attract or repel eachother :(
- All gas particle collisions are elastic ( no kinetic energy is lost )
- Gas particles are so SMALL in volume compared to the distances between the particles that the particle’s volume can be interpreted as 0 ( gases have no volume )
REAL, non ideal, gases have these caveats though:
Real gases will eventually condense into a liquid when the temp gets too low or pressure gets too high because:
- gas particles DO have attractive & repulsive forces with eachother
- Gas particles DO take up space and do have volume
Real gases behave like ideal gasses ( HT has LP )
- at high temperature and low pressure
- at these conditions, the molecules do not feel the forces between them and so they dont draw into one another and condense and solidify
Real gases Deviate from Ideal Gas Behavior
- when at high pressure, molecules are compressed, making the volume they take up more significant than if they were spread out
- when at low temperature, lower kinetic energy causes molecules to move slower and attractive forces will take place
- Polar gases ( HCl ) deviate more than nonpolar gases (He and H2)
Other properties:
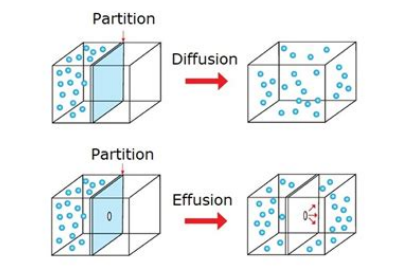
- Effusion - gas escapes from tiny hole in container under pressure
- Diffusion - gas moves across a space from high to low concentration
- As mass of particles increases, the particles move slower, the rate of diffusion / effusion is lower.
- As temperature increases, the molecules have greater average kinetic energy and will move faster. the rate of effusion and diffusion is higher.
L2 Gas Variables & Behavior
Pressure is the force produced when gas particles collide with container walls and run into a surface
Atmospheric pressure is measured with a barometer.
sea level is 1 atm or 760 mmHg. As altitude increases, the number of gas molecules decreases, and therefore so does pressure.
If temp & volume are held constant: as moles increase there are more molecules to collide with the wall, so collision frequency increases, and pressure increases.
if temp & moles are held constant: as volume increases, molecules can travel farther before hitting the wall. Collision frequency decreases and thus pressure decreases
Temperature is the average kinetic energy of a substance. As kelvin temperature increases, average kinetic energy increases, and molecular motion increases.
if moles and volume are held constant, as temperature increase, molecular motion increases so both collision frequency and force of impact increases and thus pressure goes UP
if pressure and moles are held constant: as temp increases, molecular motion increases so molecules move farther away from eachother and volume increases
if pressure & temperature are held constant:As moles increase, there will be more molecules to collide with the wall so collision frequency increases and volume increases.
Summary:
- pressure and moles direct
- pressure and volume inverse
- temp and molecular motion direct
- pressure and temp direct
- volume and temp direct
- volume and moles direct
When atm pressure > internal pressure, container implodes.

L3A: Boyle’s, Charle’s, Gay-Lussac’s Gas Laws
 1 atm = 101.3 kPa = 760 mmHg = 760 torr = 14.7 psi
1 atm = 101.3 kPa = 760 mmHg = 760 torr = 14.7 psi
temperature must always be in kelvin represented as K to convert celcius temp to kelvin use: degreecelcius + 273 = degreekelvin
standard temperature and pressure is also known as STP 1 atm ( 101.3 kpa or 760 mmHg ) and 0 degrees celcius ( 273 K )
Boyle’s law: Volume and pressure have inversely proportional relationship when temperature and moles are constant
V1 * P1 = V2 * P2
tripling pressure reduces volume by one third
as volume decreases pressure increases
as volume increases, pressure decreases
Charles law: volume and temperature have a directly poportional relationship when pressure and moles are constant
V1/T1 = V2/T2
Doubling volume doubles kelvin temperature
Gay-Lusac Law: pressure and temperature are directly proportional when volume and moles are constant
P1/T1 = P2/T2
L3B: Avogadro’s and Combined Gas laws
Avogadro’s law: Moles and volume have a directly proportional relationship when temperature and pressure are constant
V1/n1 = V2/n2
Combined gas law:

L4 Ideal and Dalton’s Law
Ideal gas law: the volume of a gas varies directly with number of moles and its kelvin temperature
P * V = n * R * T
R is the ideal gas law constant whose value is dependent on its units. R can be:
- .0821 atm L/mol * K
- 62.4 mmHg L/mol * K
- 8.314 L kPa/mol * K
use the R value that corresponds to the pressure unit in the problem
Dalton’s law: Each gas in a mixture exerts its own pressure called partial pressure. It is independent of the other gas molecules.

Modified Dalton’s law: When a gas is collected over water, the total pressure of the mixture collected is a combination of water vapor and the gas you are collecting