Electrons & The Periodic Table: Lesson 1 Check for Understanding
Bohr Models Worksheet
Part ONE: Identify the number of electrons in each energy level in the following atoms. Fill in the blanks.
| Total # of Electrons | Atom | 1st shell electrons | 2nd shell electrons | 3rd shell electrons | 4th shell electrons | Lewis Dot diagram (val e only) |
|---|---|---|---|---|---|---|
| 3 | Li | 2 | 1 | 0 | 0 | |
| 7 | N | 2 | 5 | 0 | 0 |  |
| 10 | Ne | 2 | 8 | 0 | 0 | 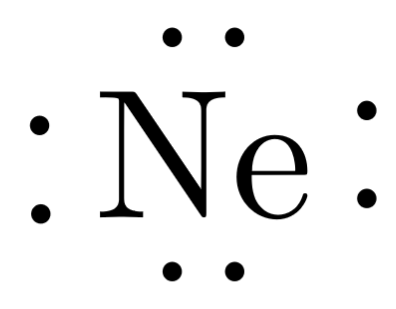 |
| 17 | Cl | 2 | 8 | 7 | 0 |  |
| 15 | P | 2 | 8 | 5 | 0 | 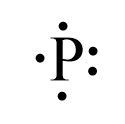 |
| 13 | Al | 2 | 8 | 3 | 0 | 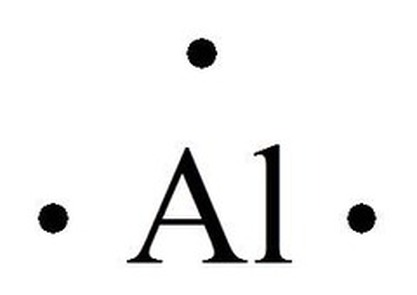 |
| 20 | Ca | 2 | 8 | 8 | 2 | 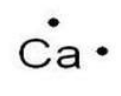 |
| 4 | Be | 2 | 2 | 0 | 0 |  |
| 1 | H | 1 | 0 | 0 | 0 |  |
| 30 | Zn | 2 | 8 | 18 | 2 | 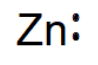 |
Part TWO: Use the information for each element to complete the diagrams. Draw the electrons into the proper shells. Place the correct number of protons and neutrons in the nucleus.
Drawing 2024-02-02 08.30.14.excalidraw