L1: What is Equilibrium?
Equilibrium is a condition where rates of opposing processes are equal. it can only take place in a closed container / space.
There are two types:
- Physical Equilibrium ( phase and solution )
- Chemical equilibrium
In phase equilibrium, the rate of one phase change is equal to the rate of the opposing phase change. This occurs when the two phases exist at the same temperature.
In Dynamic Equilibrium, molecules initially escape from the surface, and as the vapor builds up some vapor converts back to a liquid. Eventually the rate of the forward reaction equals that of the reverse reaction.
In an open container, all liquid molecules will eventually convert to a gas and leave the container.
In a closed container, the liquid converts into a vapor but remains just above the remaining liquid ( due to the closed lid of the container ) creating vapor pressure.
Vapor pressure is the pressure of the vapor above a liquid.
Factors which affect Vapor Pressure:
- IMFs
- as IMFs get stronger, Vapor Pressure gets lower.
- Temperature
- molecules have more of the energy needed to break the forces between liquid molecules
- adding a solute to a solvent
- lowers vapor pressure of a liquid
Factors which explicitly do NOT affect vapor pressure:
- Changing the container size
- Adding more of the solvent to the container
Solution ( Physical ) Equilibrium
- Rate of dissolving = rate of crystalization
- rate of the compound decomposing = rate of ions reconnecting into compounds as shown in this diagram:

- Occurs in seperated solutions
Equilibrium in chemical reactions
- Equilibrium is not reached if one of the products is withdrawn as quickly sa it is produced and no new reactants are added
- Reaction continues until reactants are used up
most chemical reactions are able to proceed in both directions under the appropriate conditions ( closed containers ) and thus are reversible.
reversible reactions are represented by a double arrow ←> in a closed system, as products are produced they will react in the reverse reaction until the rates of the forward and reverse reactions are equal
ratefor = raterev
This is called chemic* j* just mentally convert volume to pressure.
Effects of temperature changes:
- add the word heat to the correct side, then ADD AWAY, TAKE TOWARDS
* equilibrium constant is temperature dependent.
- if endothermic ( deltaH is positive ) heat can be considered a reactant
- if exothermic ( deltaH is negative ) heat can be considered a product
- removing heat favors the decrease and adding heat favors the increase. just add away take towards.
Effects of catalyst:
- Adding a catalyst increases the rate of both the forward and reverse reactions. there is no change in concentrations but equilibrium is reached quicker.
- Does not change the value of K
- just mentally convert volume to pressure.
Effects of temperature changes:
- add the word heat to the correct side, then ADD AWAY, TAKE TOWARDS
* equilibrium constant is temperature dependent.
- if endothermic ( deltaH is positive ) heat can be considered a reactant
- if exothermic ( deltaH is negative ) heat can be considered a product
- removing heat favors the decrease and adding heat favors the increase. just add away take towards.
Effects of catalyst:
- Adding a catalyst increases the rate of both the forward and reverse reactions. there is no change in concentrations but equilibrium is reached quicker.
- Does not change the value of K ust mentally convert volume to pressure.
Effects of temperature changes:
- add the word heat to the correct side, then ADD AWAY, TAKE TOWARDS
* equilibrium constant is temperature dependent.
- if endothermic ( deltaH is positive ) heat can be considered a reactant
- if exothermic ( deltaH is negative ) heat can be considered a product
- removing heat favors the decrease and adding heat favors the increase. just add away take towards.
Effects of catalyst:
- Adding a catalyst increases the rate of both the forward and reverse reactions. there is no change in concentrations but equilibrium is reached quicker.
- Does not change the value of K al equilibrium^
- Rate depends on concentration
- The forward rate of a reaction decreases over time
- Reverse rate increases over time
- Eventually they become equal.
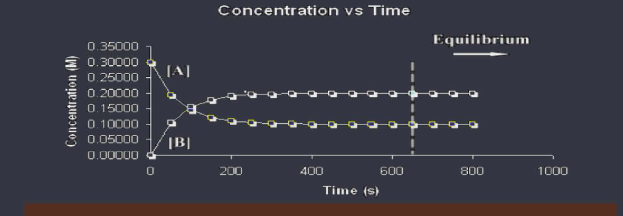
Once we have equilibrium, the amount of each reactant and product remains constant.
CON CON REQUEAL: Concentration of reactants and products are constant, but not necessarily equal. Rates are equal.
L2 Writing Equilibrium Equations
Equilibrium expression is a ratio of products over reactants Only use aqueous or gaseous substances in the expression, never use pure solids or liquids.
 lower case letter is coefficient
upper case is the elements / compounds ( chemical formula )
K is the equilibrium constant
lower case letter is coefficient
upper case is the elements / compounds ( chemical formula )
K is the equilibrium constant
If K > 1, reaction is product favored If K < 1, reaction is reactant favored
K will change with temperature only.
L3 Le Chatelier’s Principle
Whenever a stress is applied to a reaction at equilibrium, the reaction will shift its point of equilibrium to offset the stress.
Stresses include: temp changes, pressure changes, concentration changes.
Adding a reactant or product shifts the equilibrium away from the increase. Increasing product makes more reactant, increasing reactant makes more product.
Removing a reactant or product shifts the equilibrium towards the decrease. Decreasing product makes more product. Decreasing reactant makes more reactant.
Add Away Take Towards.
Effects of Volume & Pressure on gaseous Equilibrium:
- Volume and pressure have an inverse relationship
- increasing pressure ( decrease in volume ) favors the direction that has fewer moles of gas
- decreasing pressure ( increase in volume ) favors the direction that has greater moles of gas
- In a reaction where there are same moles both side, it has no effect.
- just mentally convert volume to pressure.
Effects of temperature changes:
- add the word heat to the correct side, then ADD AWAY, TAKE TOWARDS
- equilibrium constant is temperature dependent.
- if endothermic ( deltaH is positive ) heat can be considered a reactant
- if exothermic ( deltaH is negative ) heat can be considered a product
- removing heat favors the decrease and adding heat favors the increase. just add away take towards.
Effects of catalyst:
- Adding a catalyst increases the rate of both the forward and reverse reactions. there is no change in concentrations but equilibrium is reached quicker.
- Does not change the value of K