L1: Moles to count particles of a substance
1 mol of a substance = 6.022 * 10^23 “representative particles”
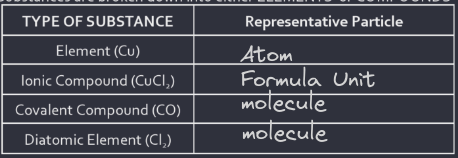
matter is broken down into substances or mixtures and substances are broken down into elements or compounds
Steps for mole conversion:
- Underline the known, circle the desired unit
- Draw a t shart and place given on the top left
- set up conversion factor in the next section: put the given unit in the denominator and the desired unit in the numerator
- match the numbers in the conversion factor to their respective units
- cancel out any units that are identical on top and bottom
- dont forget about units and round your answer to the hundredths place if possible
molar mass is the mass of 1 mole of an atom or molecule or fomula unit
also known as molecular weight, molecule mass, formula weight, and formula mass.
To find molar mass:
- Count the number of each type of atom
- Find the atomic mass of each atom on the periodic table
- Multiply the number of atoms by the atomic mass for each atom
- Add all the masses up
Molar volume of a gas is measured at STP, and 1 mol of any gas = 22.4 L
Percent Composition is the percent of mass of each element in a compound
L2 Multistep Conversions and Percent Composition
if either the known or unknown units do not contain “mol” then your calculations will be more than 1 step. the first step is to go to moles.
Finding Percent Composition:
- Add up the mass of each element within the compound to get the mass of the compound ( molar mass )
- Divide each element’s mass by the molar mass of the compound
- Multiply by 100

L3 Calculating Empirical and Molecular Formulas
Empirical formula is the simplest whole number ratio of moles to elements may or may not be the same as actual molecular formula
hydrogen peroxide has an actual formula of H2O2 and an emperical formula of HO
How to calculate empirical formula from % Comp:
- Assume 100 grams of sample. Switch % sign to grams.
- Convert mass of each element to moles of each element
- Divide all elements mole amount by the smallest amount in the entire problem. The answer is the subscript of the element within the compound.
- Optional: if the mole ratio is not within .1 of a whole number, multiply every mole amount by the smallest whole number to make it either a whole number or to within .1 of a whole number

Molecular Formula is the actual true formula of the compound. Usually a multiple of the empirical formula. N2O4 is the molecular formula; the empirical formula is NO2.
How to calculate molecular formula:
- Find empirical formula and calculate it’s molar mass. Call this EFM
- find the mass of the actual formula, which will most likely be given to you in the problem in grams. call this MFM
- divide MFM by EFM to get a factor
- multiply the factor by the empirical formula to get the molecular formula

L4 What is Stoichiometry and the MOLE RATIO
Stoichiometry is the calculations of chemical quantities from balancing chemical equations The coefficient of the balanced equation tells us how many moles or particles of eahc substance is used in the reaction A mole ratio is a conversion factor that relates 2 substances in moles. You must use a balanced chemical equation to create it. almost all stoichiometric problems can be solved in 4 simple steps:
- Do you have a balanced equation? if not, balance it.
- Are you in MOLES?
- if yes move to step 3
- if no, convert units of the given substance to moles
- using the mole ratio, calculate the moles of the given substance to moles of the substance you want.
- Possible Next Steps: Convert the moles of new substance to its desired units.

L5 Percent Yield
Percent Yield is a ratio that calculates how efficient a chemical reaction is
- The higher the % yield, the higher the efficiency, the better the reaction
- a “Yield” is a product
- Actual Yield ( A ) is the actual amount of product produced in the lab
- Theoretical yield is the amount you SHOULD produce if nothing went wrong
- Percient Yield is A/T ( Actual / Theoretical )
