Lesson 1 - Bohr Model
Basic info:
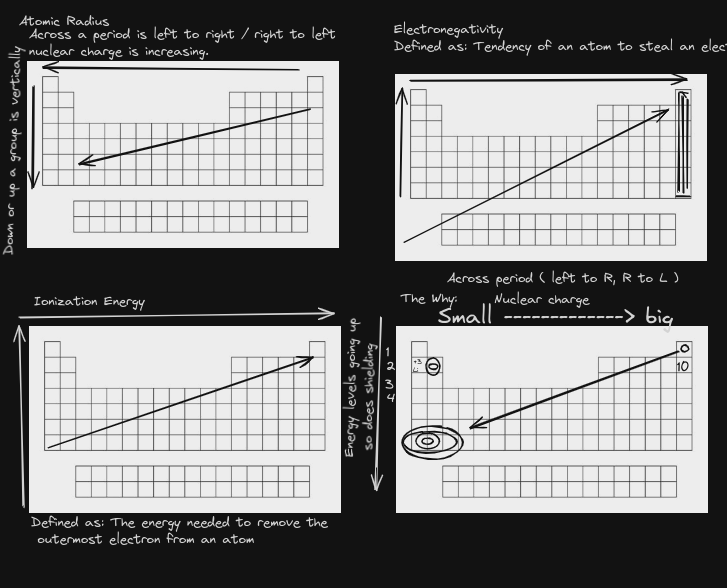
Electrons are in energy levels according to how much energy they have. only certain energy amounts are allowed in each level.
Energy levels can be thought of like rungs on a ladder, the further away energy is from a nucleus the more energy it attains
Ground state is as close to the nucleus as can get excited state is a higher energy level than it should have.
Drawing:
When assigning electrons, a max of 2 electrons are placed in the first shell, up to 8 in the 2nd shell, up to 18 in the 3rd shell, etc.
only 8 electrons can be placed in the 3rd shell at first, then 2 electrons will move into the 4th shell and the remaining of the 18 will be placed back in the 3rd shell for a total of 18
valence electrons are the outermost electrons that are found in the highest outermost energy level of the atom.
Lewis Dot Diagrams:
A way to show the number of valence electrons
2 dots per side, 4 sides, maximum of 8 valence electrons. Add dots one at a time until each side is full, then add a second dot to make a pair when needed.
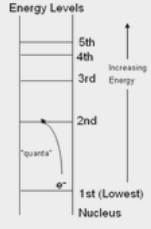
Exception is helium, which can look like this:
Quantum Mechanical model - > electron cloud model
modern atomic theory uses calculus to show how electrons act as both particles & waves
his equations show the most probable locations of electrons in an atom ( known as a atomic orbital )
Lesson 2 - Light
Electromagnetic radiation is all energy that exhibits wavelike behavior and travels through space at the speed of light, which equals 3.0 * 10^8 m/s
wavelength is the distance from crest to crest of a wave
1nm = 1 * 10^-9 m
frequency is the number of times a wave completes a cycle in one second
Notice that the shorter the wavelength, the higher the frequency, they have an inverse relationship.
As frequency increases, amount of energy increases. this is a direct relationship. the visible light region is a small segment within the spectrum which our eyes can see ( 400 - 700 nm)
Red is near infrared and violet is near ultraviolet
Sunlight will produce a range of color because there are no specific wavelengths.
A line spectrum is when individual atoms emit light of only certain, specific wavelengths. each element has its own line spectrum.
Energy packets called photons or quanta come in contact with an atom and collide with an electron.
Absorption - process of electron absorbing photon of light and being promoted to a higher energy level from its ground state. it cannot remain in that excited state forever.
Emission - the process of an electron releasing the photon of light it absorbed and falling back down to a lower energy level.
higher the energy the greater the jump. a photon of ultraviolet light would have more energy than infrared light so there would be a higher jump. Hydrogen Emission Line Spectrum - colored lines are wavelengths of light that were emitted when the electrons moved from a higher to a lower energy level. The blue line is a result of the electron moving from the 4th to the 2nd energy level.
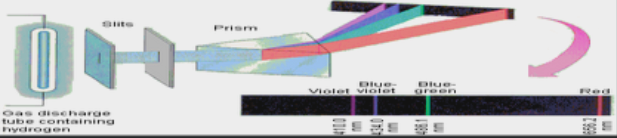
metals can be identified by the wavelenth of light they emit. When metals absorb energy from a flame, the electrons absorb energy and are raised to a higher energy level. When they return to ground state, they release their energy in the form of radiation. Wavelength of light for some metals fall in the visible light portion of the spectrum, which allows us to see their color.
Lesson 3 - Electron Configuration of Neutral Atoms and Ions
The Electron cloud as a whole can be thought of as a hotel
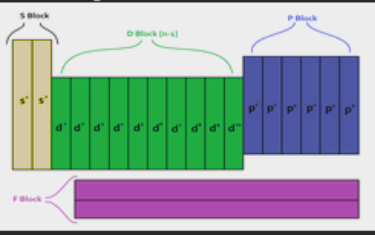
Within that electron cloud, there are 7 energy levels ( floors ). these energy levels can also be called shells. Period number on the periodic table corresponds to energy level. Within each level there are sublevels ( types of rooms ), there are S, P, D, and F sublevels. Sublevels hold orbitals. Only 2 electrons are allowed per orbital.
sublevels:
- S is spherical shaped, with only 1 orientation, which means 1 orbial. Maximum of 2 electrons. First seen in the 1st energy level. Represented on the periodic table as groups 1A and 2A + helium
- P is dumbell shaped, with 3 orientations which means 3 orbitals, maximum of 6 electrons, first seen in the 2nd energy level. Represented in the periodic table as groups 3A - 8A ( does not include He )
- D is four lobed shaped. there are 5 orientations, which means 5 orbitals. Maximum of 10 electrons. First seen in the 3rd energy level. Represented in the periodic table as group 3B - 2B
- F is too complex of a shape to name ( mrs watts calls it a flower ), there are 7 orbitals, it is first seen in the 4th energy level, and it is represented in the periodic table as the inner transition metals, lower block.
Within each sublevel are orbitals, which are areas of high probability of the electron being located. Each orbital can hold 2 electrons. To calculate the total number of electrons in an energy level, use 2n^2, with n being energy level.
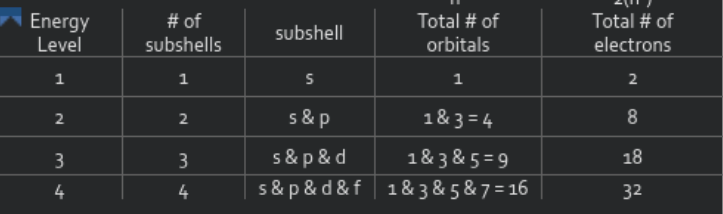
Electron configuration is an address of an electron. Electrons must be placed in the lowest possible energy levels first ( ground state ). 3 rules govern electron configuration:
- Aufbau Principle: Electrons must fill lowest available subshells and orbitals before moving to the next higher energy subshell / orbital. Periodic table shows filling order. A orbital’s cloud space can overlap within different energy levels. A 4th sublevel is lower in energy than a 3d sublevel so it gets filled first. in order to configure, determine number of electrons to place, follow aufbau principle for filling order, fill in subshells until they reach their max ( s = 2, p = 6, d = 10, f = 14). Total of all the superscripts is equal to the number of electrons.
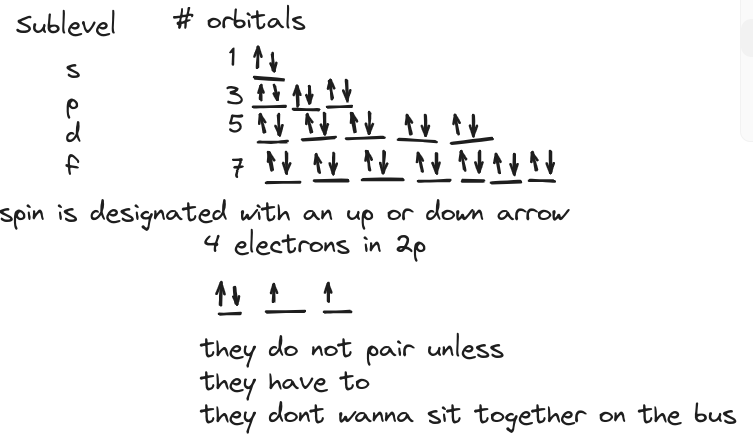
- Hund’s rule: Place electrons in each unoccupied orbital of the same sublevel before doubling up
- Pauli Exclusion Principle: Two electrons in the same orbital must have opposite spin
Orbital notation: Use boxes or lines to represent orbitals and arrows to represent electrons. Make sure to follow Hund’s rule and Pauli Exclusion Principle.
Lesson 4 - Orbital Notation & Shorthand Configurations
Shorthand Notation: A noble gas element is used to represent the core ( inner ) electrons. How to write: Determine the number of electrons to place, Determine which noble gas to use, start where noble gas is and do electron configuration like normal.
noble gasses are group 18 ( or 8a ) are noble gasses.
the reason they are noble ( or inert, meaning not reactive ) is because they
have all the valence electrons they need and dont want anymore
Lesson 5 - Periodic Table Basics
Periodic table is arranged by atomic number. Rows are periods. Columns are groups or families. Atomic number is how the elements are ordered ( equal to number of protons ). In the Element Symbol the first letter is always capitalized, and if there is a second letter it is always lowercase. Average Atomic Mass, usually with ecimals, gives the average in grams for 1 mole of atoms. This is not the mass number.
Most periodic tables have 2 rows at the bottom, which is done to allow the font to be bigger on a piece of paper, but really they belong between groups 2A and 3A.
Metals ( left of staircase ) are all solid except mercury. Metals are ductile ( can be drawn into a fine wire ) & malleable ( can be flattened into sheets ). Metals are great conductors of heat & electricity. Metals are high in luster and strength. Nonmetals ( right of staircase ) are mostly gases, few solids and 1 liquid. They have poor formability, are poor conductors of heat & electricity, and are dull & brittle.
Metalloids / semi metals ( on the staircase line, exception Al ) share properties with both metals & nonmetals. They are all solids. Some are semiconductors ( Si and Ge ), which carry an electrical charge under special circumstances. Every group ( column ) ends with the same number of valence electrons in the highest energy subshell.
2 types of elements
- Representative Elements ( main group ) ( group A )
- Transition Elements ( group B )
In group B, most have more than 1 oxidation number ( ionic charge ), they typically the least reactive of all the metals, they have the hardest, densest and highest melting points, they are malleable, ductile, and the greatest conductors of heat and electricity. main groups:
- 1A : Alkali Metals
- group number 1A
- reactive ( most reactive )
- dont occur freely in nature
- 1 valence electron in outer shell ( form +1 ions )
- soft ( low melting point and density)
- two most reactive elements: Cesium and Rubidium
- when exposed to water react violently and produce a base.
- 2A : Alkaline Earth Metals
- group number 2A
- less reactive but still reactive
- dont occur freely in nature
- 2 electrons in outer shell ( form +2 ions )
- Extracted from ores - mineral rocks
- harder, higher density, and melting points than 1A
- 7A: Halogens
- group number 7A
- nonmetals
- most reactive of the nonmetals
- term halogen means “salt former”. compounds containing halogens are called salts.
- 7 valence electrons in their outer shell
- ionic charge ( oxidation number ) is -1
- most form diatomic elements
- 8A: Noble Gases
- group number 8A
- 8 valence electrons
- non reactive
- no ionic charge
- maximum number of valence electrons. helium only hsa 2 and all others have 8.
- do not form ions.
Hydrogen is a diatomic non metal & is in a group by itself. It can have a +1 or -1 charge.
Inner transition elements are the lower block at the bottom ( 58 - 71 and 89 - 103 )
58 - 71 is lathanides, which are rare earth metals
89 - 103 are actinides, which are all radioactive and mostly man made.
Atoms try to achieve a noble gas configuration when forming an ion ( this makes them more stable ). To determine ionic charge, locate nearest noble gas and count how many “places” it is away, but remember that you can skip over the d block. The amount will be the same as the number of e’s either gained or lost by the atom when forming an ion.
Lesson 6 - Periodicity & Trends
for a period trend only - nuclear charge is the number of protons in the nucleus, determines the strength of attraction to the electrons
for a group trend only - energy level is the location of the electrons. higher the energy level the farther distance the electrons are from the nucleus.
for a group trend only - shielding is the energy levels that are between the nucleus and the valence electrons
atomic radius:
- defined as one half the distance between the nuclei of two atoms of the same element when the atoms are joined
- decreases as you go left to right ( across a period )
- as number of protons increase, nuclear charge increases, which increases the attraction between the positive nucleus and the negative electron cloud, thus pulling in the electrons closer to the nucleus to cause a smaller atom
- increases bottom to top
- the electrons are added in new, farther away energy levels. The inner electrons shield the new outer electrons from the pull of the nucleus therefore it doesnt pull so strongly THUS a large atom.
ionization energy:
- energy required to remove an electron from an atom
- increases across a period
- moving left to right, the size of an atom decreases as more protons pull harder on more electrons, when an atom is smaller, the electrons are closer to the nucleus, and therefore feel the pull more strongly. It is harder to pull electrons away from these smaller atoms thus it requires more energy to remove an electron.
- decreases across groups
- as you move down a group, the size of an atom increases as more electron shells are added. As the outer electrons ( those involved in bonding ) are farther from the nucleus, they will feel the pull of the nucleus less. it is easier to remove an electron from a larger atom thus it requires less energy to remove an electron.
Successive ionization energies:
- the energy required to remove a 2nd or 3rd electron from an atom
- removing more and more e’s requires more and more energy
- why? remaining e’s are more tightly bound to the nucleus.
electronegativity:
- the ability of an atom of an element to attract electrons when the atom is in a compound.
- Increases across a period
- when an atom is smaller, the nucleus pulls more strongly. this can attract and draw an electron shell from a different atom.
- decreases across a group
- the larger atom has weaker nuclear attractions so it cannot draw electrons away very easily from a different atom.
ionic Radii & charge:
- an ion is an atom or goup of atoms that has a positive or negative charge
- cations are positive ions resulting from a metal losing electrons
- anions are negative ions resulting from a nonmetal gaining electrons.
- ionic radius is defined as one half the distance between the nuclei of two atoms of the same ion when the ions are joined
- cations are smaller than neutral atoms
- when electrons are lost, there are now more protons than electrons. therefore, the protons have a greater “pull” on each of the electrons, thus making a smaller ion
- Anions are larger than neutral atoms.
- when electrons are gained, there are now less protons than electrons. therefore, the protons have a smaller “pull” on each of the electrons, thus making a bigger ion
- trends are same as for atomic radii.